The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public alert over a falsified pharmaceutical product, Betaclox, in the country.
NAFDAC, in an alert issued on Monday, November 10, 2025, warned Nigerians against the use of the falsified drug.
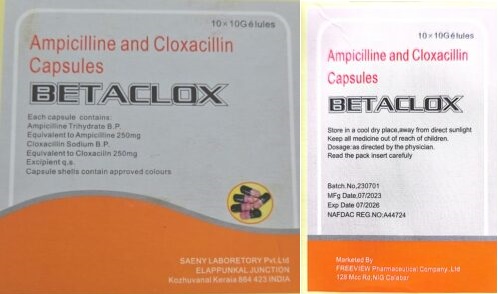
The agency disclosed that Betaclox (a combination of ampicillin and cloxacillin) contains fraudulent registration details and comes from an unverified source, posing significant risks to public health.
NAFDAC stated that the falsified product was procured from Gambori Market in Maiduguri, the capital of Borno State, by a distributor in Kano who subsequently sold it to a retail outlet in Zaria, Kaduna State.
NAFDAC said the retail outlet in Zaria reported the falsified product, leading to an investigation by the agency.
The agency said: “Preliminary investigation revealed that the NAFDAC Registration Number (NRN) A4-4724 displayed on the SF product belongs to an entirely different product: Mebendazole 500mg, manufactured by Chi Ltd. This is a clear case of misappropriation of a registration number.
Bandits kidnap five nursing mothers, steal 50 cows in Kano
“While the SF product packaging displays the address as ‘No. 128 MCC Road, Calabar,’ verification against the Pharmacists Council of Nigeria (PCN) list of registered premises confirms that Freeview Pharmaceutical Ltd. is located at ‘No. 101 MCC Road, Calabar, Cross River State.’ This discrepancy raises additional concerns about the product’s authenticity and the potential unauthorized use of the company’s name.
“BETACLOX is a combination of ampicillin and cloxacillin. Ampicillin + Cloxacillin Capsules are a fixed-dose combination drug used to treat bacterial infections of the respiratory tract, ear, nose and throat, urinary tract, skin and soft tissue, and gastrointestinal system.”
NAFDAC added that all the agency’s zonal directors and state coordinators have been directed to carry out surveillance and mop up the falsified product across the country.
“Distributors, retailers, healthcare professionals, and caregivers are hereby advised to exercise caution and vigilance within the supply chain to avoid the distribution, sale, and use of the SF product. All medical products must be obtained from authorised/licensed suppliers. The products’ authenticity and physical condition should be carefully checked,” the agency stated.
- 2 injured as drug traffickers attack Customs officers in Ogun - January 25, 2026
- Nwaneri scores on Marseille debut as Lens lose Ligue 1 top spot - January 25, 2026
- Governor Yusuf sacks political adviser, names replacement - January 25, 2026