Co-Chair, Bill & Melinda Gates Foundation
A decade of research into why kids in the poorest places die is now unlocking ways to save millions
It’s been almost a decade, but it’s still hard for me to tell this story without choking up.
It was 2016, and I was visiting a hospital in Johannesburg, South Africa—specifically, Soweto, the township on the city’s outskirts, which had some of the highest child mortality rates in the world.
Even at wakes and funerals it’s sometimes shocking for me to see a dead body, but this didn’t look like any dead body I had seen before. It was so small, covered in plastic. Only after I stepped closer did I recognize it was a newborn baby, maybe a day or two old.

Afterwards, I collected myself and went outside. The child’s parents were there.
I had met parents who’d lost children before, but not like this. When children died in poorer countries, they were never brought to a hospital or a morgue. Sometimes, a health worker would travel to the home and ask what had happened, but medical examiners and doctors didn’t perform an autopsy—not until CHAMPS.
Child Health and Mortality Prevention Surveillance, CHAMPS, is an initiative our foundation started in 2015. The idea was to learn more about the root causes of child death by taking blood and tissue samples from children who’d died, but no one was quite sure whether, on the worst day of their lives, the parents would agree. The couple outside the Soweto hospital were among the first to volunteer, and I wanted to learn why.
“We just don’t want this to happen to another family,” they told me.
That’s what sticks with me—not simply the tragedy, but the kernel of hope. Those parents saw a bigger picture on the day their greatest fear was realized. And it was up to the rest of the world to do better by them and millions of parents like them: We had to find out why children were dying to keep them alive.

Even ten years ago, public health officials had only the vaguest information about why babies were dying. Back then, any record of a child’s death would generally list one of the four most common causes: diarrhea, malnutrition, pneumonia, or premature birth. But each was a vast ocean of different illnesses, with scores of different causes and cures. Pneumonia, for example, is linked to more than 200 types of pathogens.
Answering “Why did a child die?” felt a little bit like being asked to find a child lost at sea—except you were only told which ocean to search in, Atlantic or Pacific. There was an expanse of missing information, so our foundation decided to help fill that void by funding research including three landmark studies. In addition to CHAMPS, which was aimed at explaining the most inscrutable causes of death, there was also the Pneumonia Etiology Research Child Health Study, PERCH, which examined the causes of childhood pneumonia, while the Global Enteric Multicenter Study, GEMS, did the same for diarrheal diseases.
As doctors compiled and compared case after case, a clearer (and often surprising) picture of child death emerged. For instance, some pathogens were less likely than was expected, like pertussis, which causes whooping cough, but others were more likely than we expected, like Klebsiella, which can be harder to treat.
Imagine if doctors didn’t know why American men were susceptible to heart attacks—and then, in the span of two years, they discovered the links to high cholesterol and smoking. That’s what happened with infant pneumonia, and the new information about Klebsiella is leading doctors to change what antibiotics they use.
More precise understanding of why children die
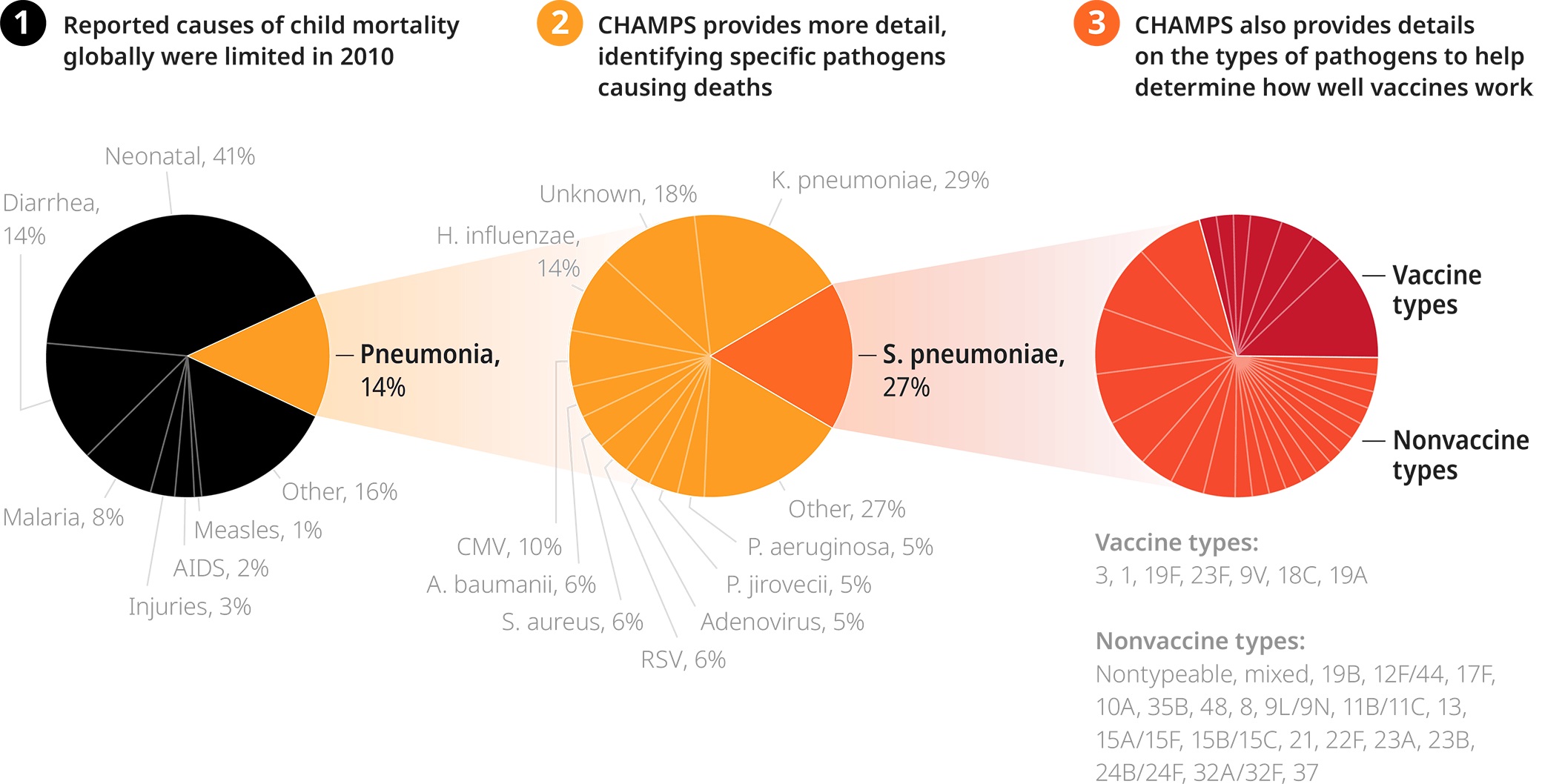
CHAMPS data provides hyper-detailed information about which pathogens are causing deaths, guiding the development of improved treatments and vaccines.
This is the crux of what I call “the baby knowledge boom.” Thanks to studies like CHAMPS, GEMS, and PERCH, the medical field has begun to understand precisely when and why some babies are dying, which allows them to keep others alive.
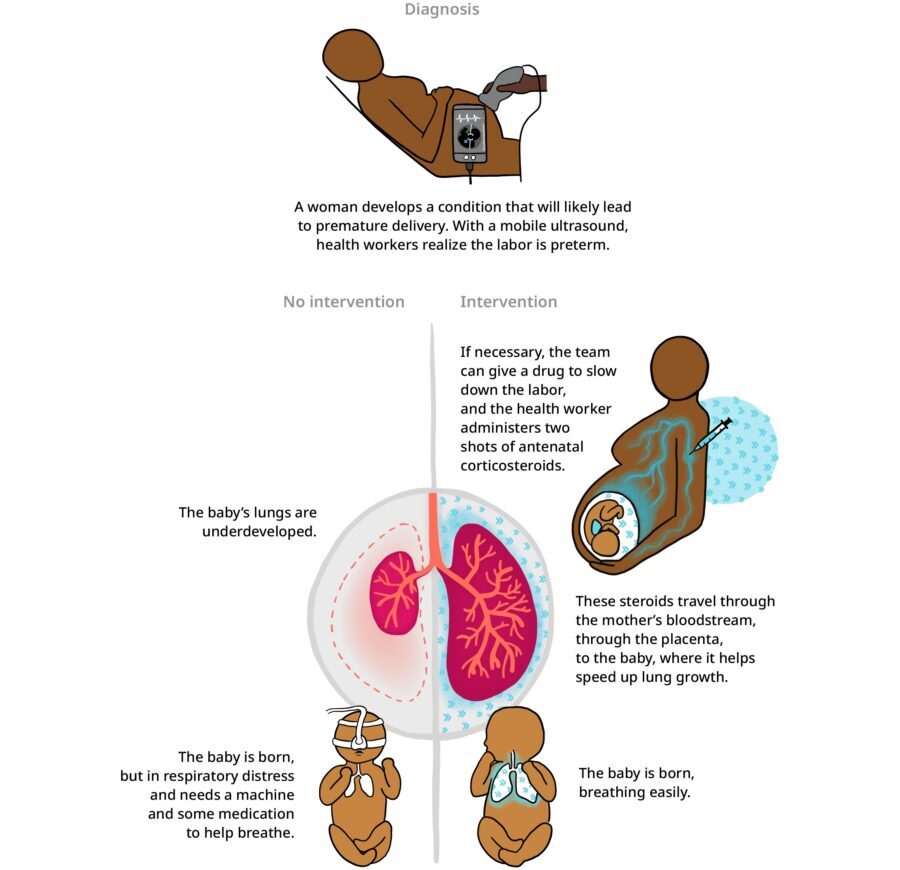
Another great example is how doctors are helping premature babies breathe—by using novel methods to “fast-forward” their lung growth. If a doctor sees that a mother is going to give birth prematurely, they can give her antenatal corticosteroids, or ACS. The ACS “exercises” the fetus’s lungs, which accelerates their growth, packing a few weeks’ worth of maturation in just a few days. Our foundation estimates that ACS could save the lives of 144,000 infants in sub-Saharan Africa and South Asia by 2030 and nearly 400,000 by 2040.
But that’s just a fraction of the lives we can save if we apply what researchers learned about nutrition in the past decade.
Antenatal corticosteroids fast-forward baby’s lung growth
Gut check
If you’ve seen medical TV shows like CSI or House, MD, you already have some sense for how an initiative like CHAMPS works. Doctors and pathologists sit on a “decode panel,” reviewing cases, batting ideas back and forth, until they come to a full conclusion on all the steps that led to a person’s death.
This level of detail is important because, outside of unexpected accidents, few people die for just one reason. Instead, death is a chain reaction. For example, a baby who dies of pneumonia likely wasn’t just fine before getting sick. She likely was born premature or was undernourished. The best way to keep a child alive isn’t to treat the pneumonia that will kill her. That’s a last resort. Rather, we should try to stop the first link in the causal chain from being formed in the first place.
Studies like CHAMPS helped us understand that often that first link is malnutrition.
Believe it or not, I think this is positive news. Because our growing understanding of why children die has proceeded alongside a second, arguably bigger knowledge boom—this one involving our grasp of nutrition.
If we’ve packed 100 years of learning about maternal and child mortality into the past decade, researchers have probably crammed 1,000 years’ worth of knowledge about the microbiome in the same decade, which is the teeming universe of bacteria that lives inside our digestive tracts. For example, the child health field used to think of breastmilk as only food for the newborn. But we’ve now learned that it’s also food for the bacteria that naturally live in the gut of the baby.
These bacteria—the most common ones are called bifidobacteria—break down specific sugars in the milk, turning it into nutrients. Without these good bacteria it doesn’t matter how well you feed your baby; their digestive system would still have a really hard time absorbing the milk’s nutrients. Which is why doctors are now recommending that babies—especially those born too soon or too small—are given a probiotic supplement with bifidobacteria in it.
B. infantis improves baby’s gut microbiome
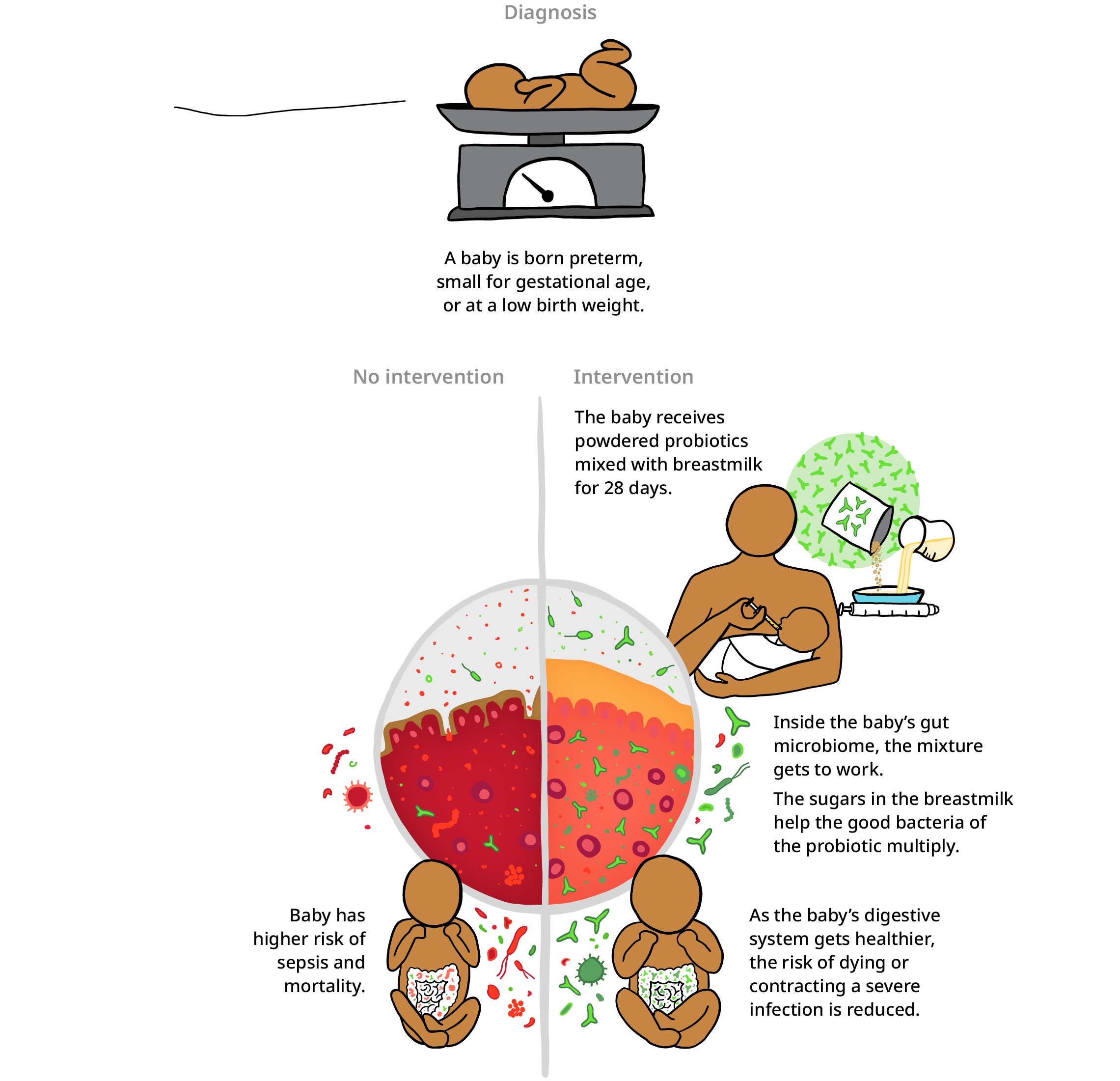
This next part is gross, but amazing. Bifidobacteria are different depending on where you’re from. Babies in India have different gut bacteria than babies in the United States, so these probiotics have to be tailored hyper-locally—or in this case, “diaper locally.” Researchers swab the poop of a baby, isolating the bacteria that live in their intestines, then analyze the unique way they work in their digestive tract and can create locally tailored probiotics based on that research.
There are other new supplements to fight malnutrition, but maybe the biggest innovation is when doctors are providing them: in the womb. The medical field used to think that you couldn’t treat malnutrition until a child was about six months old and started eating. But new research has found that the baby’s microbiome and the mother’s are connected. If a pregnant woman has abundant bifidobacteria, good bacteria can spread from her digestive tract to the child’s; this way, the baby is born already having a healthy gut.
Studies show these probiotics help babies gain an additional 5 grams of weight per day in the late stages of pregnancy and can improve baby’s growth when given to the baby after birth.
Delivering healthy babies and saving millions of lives
Low-cost innovations can prevent millions of stillbirths and infant deaths in LMICs.
Remembering Soweto
“We just don’t want this to happen to another family.”
What those parents in Soweto said to me has echoed in my mind for over seven years, and I’ve often wondered how I might respond if I saw them again.
I think I would be honest. It might not be possible to protect every family, to guarantee a world of zero newborn deaths. Zero is a hard number.
But that doesn’t mean we can’t come very, very close.
Over the past decade, the field of child health has moved faster and farther than I thought I’d see in my lifetime. And if our delivery can keep pace with our learning—if researchers can keep developing new innovations and health workers can get them to every mother and child that needs them—then doctors could all but guarantee a baby would survive their crucial first days.
That’s what I would tell them. That’s what, together, I believe we can show them.
“Over the past decade, the field of child health has moved faster and farther than I thought I’d see in my lifetime.”
- Alex Otti should publish Abia Forensic Report now - May 1, 2024
- FG approves 25%, 35% salary increment for civil servants - April 30, 2024
- CBN director: How I collected $600,000 contract bribe for Emefiele - April 29, 2024










